Chlorine Water Treatment for Drinking Water

Water treatment can be a challenge in an emergency and here is the why and how chlorine will be effective in the process of creating drinking water
For purposes of water purification, chlorine will be your friend – especially in an emergency scenario where normal utilities like power, water and sanitation are compromised either for a short period (few days to weeks) or outages lasting for a lengthy but undetermined time. Public water supplies are usually the first to become contaminated in times of natural disasters or emergencies. Chlorination has been in public use in the United States since 1906 when it was introduced to municipal water supplies as a disinfectant. Up to that time, serious illness, and death due to drinking water contaminated with pathogenic microbes was common in both first world and third world countries alike. Since the introduction of chlorine to public water supplies, the mortality rate from drinking water has fallen drastically, especially in first world countries where tap water is deemed safe to drink.
Chlorination of water supplies for water purification has been proven safe and effective through numerous scientific studies and over the course of time. The Environmental Protection Agency (EPA) regulates public water systems and deems water safe to drink up to 4 parts per million (ppm) of chlorine. You can easily measure chlorine concentration using test strips or using inexpensive kits you can obtain online or at pool supply stores.
Chlorine is also effective in sanitizing and disinfecting areas involved in food preparation as well as cleaning and sanitizing surfaces that may come in contact with human waste.
One of the advantages of chlorination is that not only does it kill microbes and organic compounds on contact, but it also has a residual effect meaning that as chlorine persists for a time in treated water, it will continue to disinfect if any new bacteria or viruses are introduced.
Chlorine disinfection methods are inexpensive and readily available to consumers. For those who are sensitive to chlorinated water or just don’t like the taste, the concentration will be significantly reduced via a water filtration system using an activated charcoal filter, through aeration of the free chlorine water, and/or extended exposure to sunlight in the open air.
Chlorine is very effective to kill bacteria, viruses and waterborne diseases but doesn’t kill single cell protozoa like cryptosporidium.
Water chlorination will not be effective, however, in killing organisms such as cryptosporidium. These single celled protozoa reproduce in the gut of a host (mammals and birds) and then the eggs are shed as a tough shelled cyst (which is resistant to chlorine, bromine, or iodine) when the host defecates. These cysts can persist in the environment for quite a long period. Once ingested, they become active and reproduce this life cycle in the new host—often making it quite ill especially in humans. Because these cysts are relatively large (1 to 3 microns), micro-filtration presents an effective means of removing them from a water supply. Boiling is also effective as is ultra-violet (UV) treatment. It is estimated that up to 80% of the rivers and lakes in the U.S. are contaminated with these microbes.
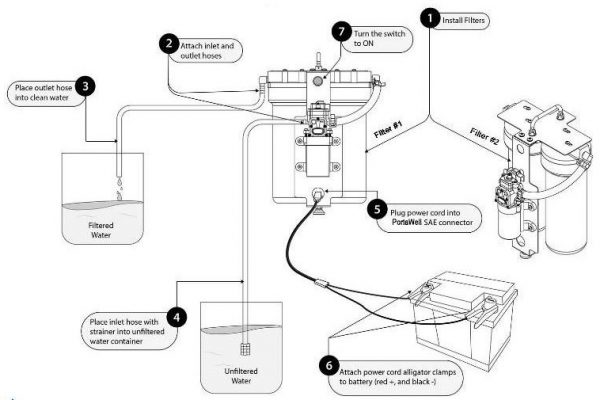
This reason for both micro filtration and chlorination should be considered in making surface water suitable for drinking, especially when the water is suspected of significant viral contamination or heavy concentration of disease-causing microbes (slow moving stream, pond or other heavily clouded water).
Commercial chlorine bleach has a shelf life and chlorine concentrations are important
There are precautions to be taken when using chlorine to disinfect your own water supply. An important question is:
How much chlorine do I add to disinfect the water and still be safe to drink?
In answer to this question, it depends on:
- chemistry of the chlorine source,
- the concentration of the chlorine source and
- the age of the chlorine source.
For instance, a common source of chlorine mentioned in many internet searches for disinfecting water is household liquid bleach. Commercial bleach that you buy at a grocery store is sodium hypochlorite (NaOCl). Numerous companies make and market this product. Some add chemicals to make the smell more pleasant, some add thickeners to reduce the likelihood of splashing while pouring, and they also make different concentrations—from 5% up to 10% concentration.
If using household bleach as a disinfectant, you should choose a product that has no additives for smell or thickening, you should know the chlorine concentration and you should know the manufacture date to know the age of the bleach.
It is important to know that liquid bleach loses its potency over time. Shelf life for liquid bleach is generally recognized as 1 year.
Because liquid chlorine bleach has a short shelf life consider a different water purification chlorine source
There is a better solution to chlorinate water to make it suitable for drinking, and this will be to use calcium hypochlorite (Ca(OCl)2 ) instead of household bleach (sodium hypochlorite).

Calcium hypochlorite is:
- a powder rather than a liquid,
- it readily dissolves in water,
- (if stored properly) it has a shelf life up to 10 years,
- inexpensive and available, and
- very concentrated so a little goes a long way.
A common use for calcium hypochlorite is pool shock—used to control algae bloom in swimming pools. You can purchase it either online, from pool supply sources or sometimes from your local preparedness store. It is a potent chemical so take care how you store it and use it. If you follow the precautions on the label, it is perfectly safe. Just remember it eats metal and fumes will be strong so store it in a closed plastic or glass container, in a cool location, out of direct sunlight and use in a well-ventilated area. The brand “Drytec” (figure 1) claims to be safe for treating drinking water. Once it is in liquid format it also has a 1-year shelf life.
Because Calcium Hypochlorite is so concentrated (usually 65 to 70% chlorine by weight), it is recommended to first mix your own “chlorine bleach solution” (say 1 gallon at 600 ppm) and then use this lower concentration liquid solution for final treatment of your drinking water. This two-part approach ensures a better dilution accuracy and ensures that the “bleach” solution is at full strength when you use it
Table 1 below represents the two-part mixing strategy.
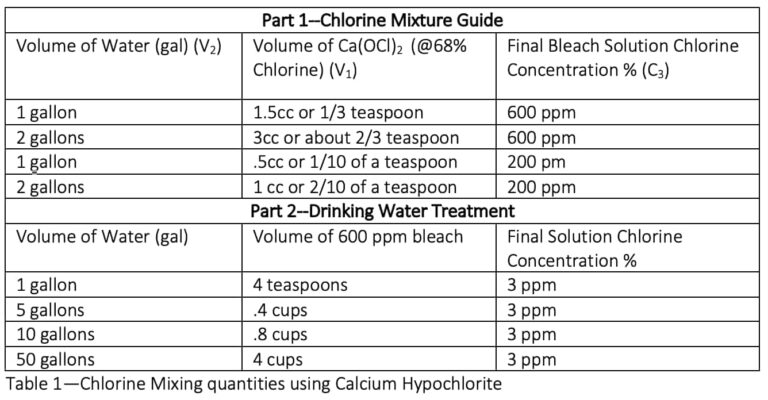
Note: A 600 ppm solution can be used for disinfecting food prep surfaces and a 200-ppm solution for sanitizing eating dishes and utensils
Appendix A below gives the mixture formula for developing your own custom table and Appendix B and C gives example problems on how to use the formulas.
When should I treat my water with chlorine?
In a typical municipal water treatment plant, chemical treatment (chlorination) is the final drinking water treatment step before the water is stored and delivered to the consumer.
The reason for this is two-fold.
- A high concentration of organic particulate matter adds significant uncertainty to the correct chlorine dosage to make the water suitable for drinking. If the water is treated with chlorine before removal of this particulate, it requires more chlorine. There may not be enough active chlorine left to completely disinfect the water to drinking water standards. This is because most of the chlorine can be used up reacting with the organic particulate rather than disinfecting the pathogenic microbes.
- Chlorination of water with a high concentration of organic particulate will result in a higher concentration of treatment byproducts such as trihalomethanes. This can present long term negative health effects. Trihalomethane is an EPA regulated drinking water contaminant.
For these reasons it is best practice to chlorinate after the water has been filtered for removal of visible particulate. This practice:
- minimizes the amount of chlorine necessary to add,
- improves the accuracy of the dosage calculations,
- minimizes the creation of potentially harmful byproducts, and
- provides a residual chlorine concentration for disinfection of any microbes in your storage containers.
In summary:
In an emergency scenario where clean drinking water no longer comes out or your tap, you have exhausted your supply of stored water and you must turn to available surface water to survive:
- A combination of microfiltration (PortaWell) followed by chlorination should be considered, especially if source water is suspected of viral contamination or heavy concentration of disease-causing microbes (slow moving stream, pond or other heavily clouded water).
- Mixture concentrations are easy to calculate using Table 1 and the simplified formulas listed in Appendix A.
- Powdered calcium hypochlorite is a better storage option for chlorine treatment of water or disinfection, sanitation, and drinking than common bleach (sodium hypochlorite) because it:
- Has a much longer storage shelf-life
- Better ability to predict and calculate more precise dosage because of known concentration.
- The powder is very concentrated and occupies a smaller storage footprint.
- Is economical and readily available.
- Chlorination (if needed) should be applied after filtration to:
- Minimize and amount of chlorine required.
- Minimize to formation of potentially harmful chlorinated by-products.
- Provide residual chlorine in stored water to reduce potential for algae buildup.
Note: Chlorine can always be removed or significantly reduced from water prior to drinking by filtering the chlorinated water through an activated charcoal filter.
Appendix A—Mixture formulas for adding chlorine to water
Formula 1–Solid-Liquid Mixture—Adding powdered calcium hypochlorite to pure water to make a stock “bleach” solution (Part 1 in table)
V3 = 1607 * V1 * C3/C1
Where:
V3 is volume of concentrated chlorine solution to add in cc (cubic centimeters)
V1 is the volume of water being treated in gallons.
C3 is the desired chlorine concentration in ppm (parts per million) of the treated water.
C1 is the concentration of the concentrated chlorine solution in ppm (1% = 10,000 ppm)
Formula 2–Liquid-Liquid Mixture—Adding stock “bleach” chlorine solution to untreated water (Part 2 in table)
V3= 768*V1*C3/C1
Where:
V3 is volume of concentrated chlorine solution to add in teaspoons.
V1 is the volume of water being treated in gallons.
C3 is the desired chlorine concentration in ppm (parts per million) of the treated water.
C1 is the concentration of the concentrated chlorine solution in ppm (1% = 10,000 ppm)
Appendix B—Example problem mixing powdered calcium hypochlorite to water
How much powdered calcium hypochlorite do I add to 1 gallon of water to make a solution of 600 ppm chlorine if calcium hypochlorite has a concentration of 68% chlorine by weight. (Part 1 in table)
Here V1=1 gallon; C3= 600 ppm and C1=68 %
Converting C1 to a ppm=68 *10000 or 680000 ppm and making other units consistent
Using Formula 1
V3 = 1607 * V1 * C3/C1
Note: the constant 1607 results from conversions to make measurement units consistent
V3 = 1607 * 1 * 600/680000
V3= 1.42 cc (cubic centimeters) (1 tsp = 5 cc)
Therefore adding ~ 1.5 cc (or 1/3 tsp) of 68% calcium hypochlorite will treat 1 gallon of water to a concentration of 600 ppm chlorine.
Note: The 600-ppm solution is also good for disinfecting surfaces that may come in contact with harmful microbes. A 200-ppm solution is recommended for sanitizing dishes and eating utensils. It is easy to convert the 600-ppm solution to 200-ppm by adding 2 parts water to 1 part of the 600-ppm bleach solution.
Appendix C—Example problem mixing 600 ppm stock bleach solution to water
How much stock bleach solution with a concentration of 600 ppm do I add to 25 gallons of water to treat it to the EPA maximum recommended concentration of drinking water of 4 ppm? (Part 2 in table)
Here V1=25 gallon; C3= 4 ppm and C1=600 ppm
Using formula 2 above
V3 = 768 * V1 * C3/C1
Note: the constant 768 results from conversions to make measurement units consistent
V3 = 768 * 25 * 4/600
V3= 128 tsp (1 cup = 48 tsp)
128/48= 2.2/3
Therefore adding 128 teaspoons or 2 2/3 cups of stock 600 ppm bleach solution will treat 25 gallons of water to a concentration of 4 ppm.
Categories
Facebook
Twitter
Email
Print
Recent Posts
Water Sustainability: Powering Your PortaWell® System
Sustainability is a key element especially when it comes to water and PortaWell can help provide sustainable water in an emergency. Clean water is required ...
Read More
Selection of Surface Source Water
One PortaWell® customer asked the question: I live near the Jordan River in Salt Lake City, Utah. Could I use it as a water source ...
Read More
Sustainable Water Solution for Emergency Preparedness
sus·tain·a·bil·i·ty – Noun – the ability to maintain or support a process over time. Sustainability, as it applies to emergency preparedness, is particularly important if ...
Read More
Filtering Pool Water
Filtering Pool Water – PortaWell® Many people have inquired if they can filter pool water with a PortaWell® system to make it safe for drinking ...
Read More
PortaWell® Newsletters
PortaWell® Newsletters Download and read our past newsletters with tips and tricks for using your PortaWell®: Water Filter Placement and Selection – Newsletter Volume 1 ...
Read More
PortaWell® System Operating instructions
Congratulations on your purchase of the PortaWell® Emergency Water Filtration System. This patented system (US 11,274,048 B2) is made in the USA and is designed ...
Read More
PortaWell® Products
-
Argonide NanoCeram® Filter (10-inch, 0.2 micron)
$52.99Original price was: $52.99.$43.95Current price is: $43.95. -
PortaWell® Starter Pak
$379.00Original price was: $379.00.$339.00Current price is: $339.00. -
PortaWell® Mini Expedition
$279.99Original price was: $279.99.$249.00Current price is: $249.00. -
PortaWell® Expansion Kit
$165.00Original price was: $165.00.$149.00Current price is: $149.00. -
PortaWell® Plus Kit
$539.00Original price was: $539.00.$479.00Current price is: $479.00. -
PortaWell® Survivor Kit
$325.00Original price was: $325.00.$289.00Current price is: $289.00. -
PortaWell® Solar Accessory Kit
$71.95Original price was: $71.95.$59.95Current price is: $59.95.